Soap films are fascinating not just for their beauty but also because they are a powerful way to study complex fluid dynamics in a simplified form. In this experiment, we use soap films to explore two major concepts in physics: interference of light and fluid flow behavior, specifically the formation of vortex streets. Soap films are often seen as a 2D fluid system because they are skinny, which makes them an ideal medium for studying fluid flow and other phenomena that might be difficult to visualize in three dimensions.
Physics Behind Soap Films
A soap film consists of a thin layer of water trapped between two layers of soap molecules (surfactants). The water is pulled by surface tension, and the soap molecules stabilize the film by reducing the surface tension, making it more elastic. This balance between surface tension and elasticity keeps the soap film intact and prevents it from breaking into droplets. The soap molecules are amphiphilic molecules with a hydrophilic head and a hydrophobic tail.
Surface Tension and Stability
Surface tension is the force that causes liquid surfaces to contract, making the surface area as small as possible. For soap films, this force tends to make the film shrink. However, the soap molecules, known as surfactants, reduce the surface tension enough to stabilize the film. Without this stabilization, the thin water layer would break apart. The film’s thickness can range from several micrometers to as thin as 100 Å (angstroms). Over time, as water evaporates, the film becomes thinner, eventually forming a "black film" where interference is no longer visible.
The Science of Soap Solution
A soap film's stability depends on the soap concentration in the water. Soap reduces surface tension, making the film easier to form, but if the soap concentration is too low, the film becomes unstable and breaks. On the other hand, too much soap can make the film thick and less responsive to interference patterns. Our experiment found that a 5% solution of dishwashing soap (Vim) with a few drops of glycerin produced the most stable film. Glycerin helps by slowing down evaporation and adding elasticity to the film.
Experimental Setup
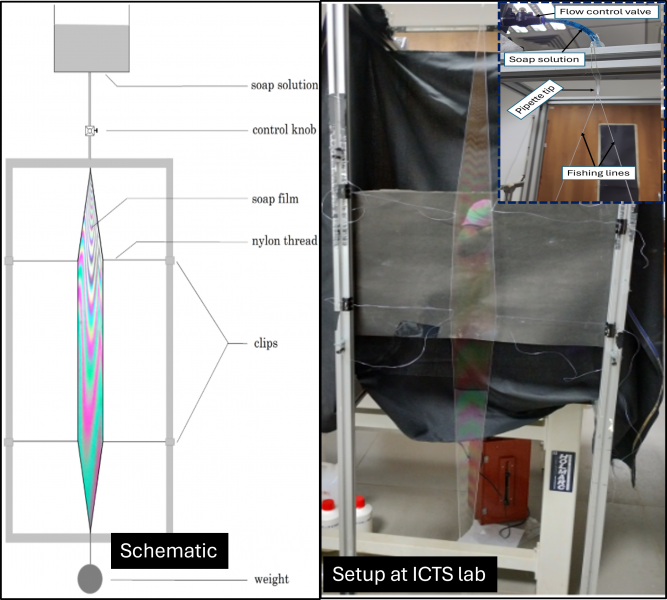
Soap film set-up implemented at ICTS
To observe the soap film, we used a setup where thin two nylon threads (fishing lines) were stretched between two points after coming out from a hole of a pipette tip through which the soap solution is flowing from a soap solution reservoir placed at a certain height. The soap film forms between the threads. We then allowed the soap solution to flow under gravity by controlling the flow with a knob, which allowed us to stabilize the film and study both the interference patterns and the fluid flow phenomena.
Interference of Light in Soap Films
When light hits a soap film, part of it is reflected off the top surface, and part of it travels through the film and reflects off the bottom surface. These two sets of light waves then interfere with each other either constructively (adding up to make brighter light) or destructively (canceling each other out), depending on the thickness of the film. This interference creates the colorful patterns we observe. See interference patterns observed with white light at our lab below.
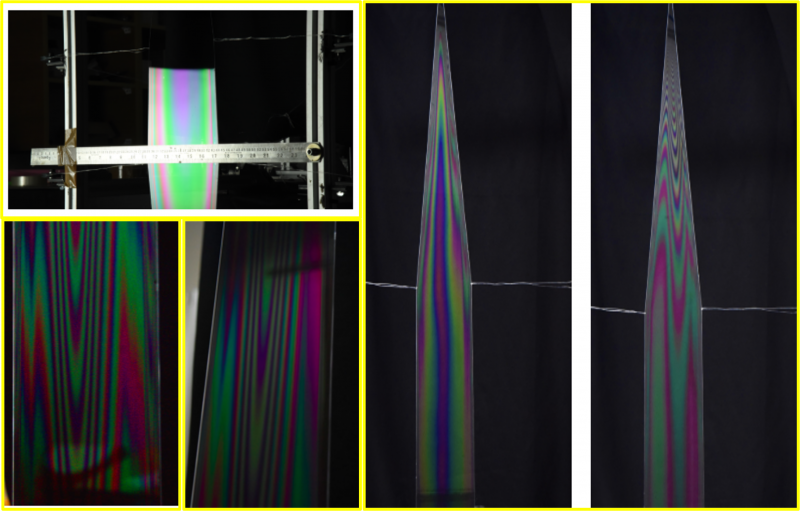
Interference of light captured employing a white light at our lab
Thin Film Interference
The colors of soap films come from thin-film interference, which occurs when the thickness of the film is comparable to the wavelength of light (in the range of visible light: 400 nm to 700 nm). As the film gets thinner, the colors change because the path difference between the light reflected from the top and bottom surfaces also changes. Eventually, when the film becomes extremely thin (around 50 Å), it appears black because the destructive interference completely cancels out the reflected light and known as Newton's black films (see images below).
Black films captured at ICTS lab
Fluid Flow and Von Kármán Vortex Street
Beyond the colorful interference patterns, soap films also provide a unique way to study fluid flow in two dimensions. One of the most intriguing phenomena we can observe in this setup is the Von Kármán vortex street, a repeating pattern of swirling vortices that form when fluid flows past an object. This happens at specific ranges of the Reynolds number (a dimensionless number that characterizes fluid flow). We have used a monochromatic Na light source, a high-speed camera (fps > 2000), and a semi-dark room to observe the VK streets.

Set-up at the lab for vortex street visualization
Von Kármán Vortex Street
When fluid flows past an obstacle, like a cylindrical rod, alternating vortices are shed from opposite sides of the object, creating a repeating pattern downstream. This is known as the Von Kármán vortex street. The formation of these vortices depends on the Reynolds number. As the Reynolds number increases beyond a critical value, these vortices become more organized and stable, forming a regular pattern. Below this threshold, the flow remains smooth and steady.
In our experiment, we used cylindrical rods of different diameters (2.2 mm, 4.4 mm, etc.) to observe the formation of vortex streets (see the images below). The smaller rod produced tighter, more closely spaced vortices, while the larger rod produced broader, more widely spaced vortices.

Von Karman Vortex street recorded with a high-speed camera at ICTS
The Effect of Temperature on Vortex Formation
Temperature affects the viscosity of the soap solution. As we increased the temperature of the cylindrical rod and placed it into the soap film, we observed that the Von Kármán vortex street became distorted. This is because higher temperatures reduce the viscosity of the fluid, making the flow more chaotic and less stable. This highlights the delicate balance between fluid properties and flow behavior.
Resources:
Acknowledgments: This experiment has been successful with the help of integrated PhD students at ICTS, Prof. Mahesh Bandi (OIST), Prof. Rama Govindarajan, & Prof. Abhishek Dhar (ICTS)


